Research
How do proteins shape, interact and function in a living cell?
Coordinate of “structured” and “non-structured” regions in protein function and behavior
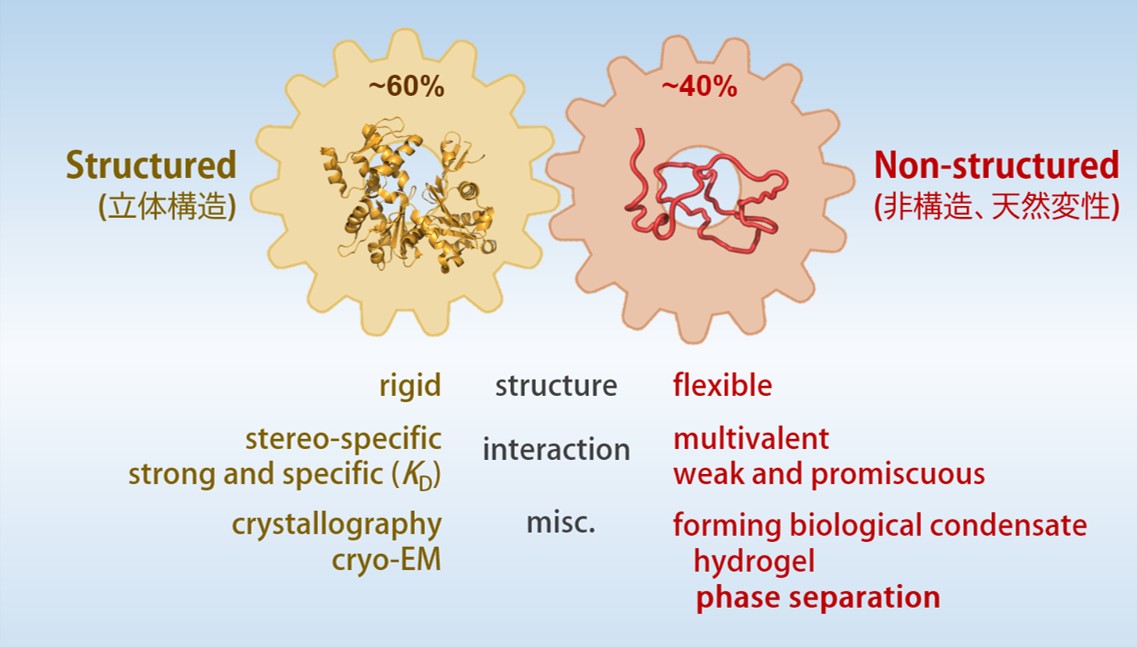
Conventional molecular biology and biochemistry has been established on the basis that “the specificity of protein function is affirmed by its three-dimensional structures”. Three-dimensional structure of protein enables specific enzyme-ligand and protein-protein interactions even in a crowded environment of intracellular milieu.
However, many studies using proteomics and bioinformatics found that protein domains with three-dimensional structures occupy only 60% of human proteome and 40% are disordered (Intrinsically-Disordered Region, IDR). The function and the structural properties of IDR has been studied but remained elusive for many years. Recent studies, however, demonstrated that IDRs tend to form hydrogel and/or undergo liquid-liquid phase separation (LLPS), which play critical roles in structural and functional dynamics of intracellular membrane-less organelles. IDRs are now widely recognized as an essential part of cellular proteins and necessary for understanding the mechanism of life.
Our group is keen for elucidating the structural and functional properties of IDRS and understand their roles in various cellular events such as cell cycle regulation, intracellular signaling, post-translational modifications, and innate immune system. We hope to find a novel mechanism of protein function and behavior, which can not be described by conventional molecular biology.
Research Projects
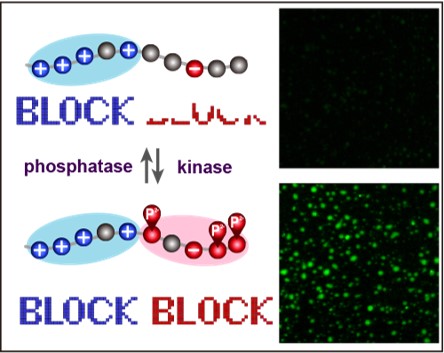
Regulation of “charge block”–driven LLPS by phosphorylation
LLPS are driven by several different types of interaction such as electrostatic, hydrophobic and cation-pai. Our group reported that “charge-block”-driven LLPS, and its regulation by post-translational modifications play critical roles in the structural and functional regulations of membrane-less organelles. This mechanism could answer a number of unsolved questions on protein phosphorylation.
[2020 BBA Prot. Proteom. / 2021 FEBS Lett. / 2022 Nat. Cell Biol. / 2023 生化学 / 2023 生物物理]
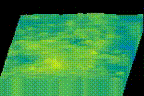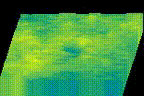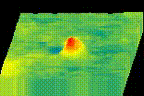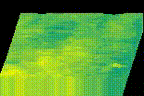
How protein LLPS deforms plasma membrane during endocytosis
The surface of biological membrane enhances self-assembly and LLPS of proteins and could induce various membrane deformations. We combine various live-cell imaging techniques to visualize and analyze the molecular mechanism of clathrin-mediated endocytosis. We are especially interested in how LLPS on the plasma membrane accelerates the pit assembly and membrane fusion.
[2018 PLOS Biol. / 2021 J. Cell Sci. / 2022 bioRxiv]

How IDRs form a “selective barrier” within the nuclear pore complex
Macromolecular transport between cytoplasm and nucleoplasm is mediated by nuclear pore complex (NPC) in the nuclear envelope. The central channel of the NPC comprises of a number of disordered proteins and works as a selective barrier for various macromolecules. We tried to elucidate how such barrier is formed and how molecules go through the barrier.
[2008 PNAS. / 2013 J. Cell Sci. / 2014 Structure / 2016 Mol. Biol. Cell / 2017 Sci Rep. / 2020 FASEB J. / 2020 Cell Rep.]
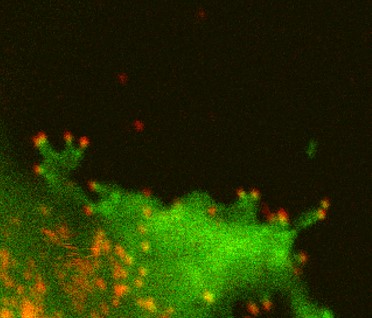
LLPS in host-virus interaction
Viral proteomes are known to contain a larger fraction of disorder regions. This is considered as a viral strategy for efficient usage of limited genome and protein resources. We are interested in how viral proteins interact with host factors via LLPS. We are especially interested in anti-viral host restriction factors which contains a large disordered region and inhibits the production of HIV-1.
[2021 Viruses]

Analyzing mechanical properties of cell cortex in multi-cellular system
We have been utilizing various AFM-based techniques (live-cell HS-AFM and force measurement) to comprehensively understand the mechanical properties of the cell cortex and morphological dynamics of the plasma membrane. We now try to apply these techniques for multi-cellular systems such as embryogenesis and tissue morphogenesis.
[2008 J. Microscopy / 2008 PNAS. / 2017 Microscopy / 2018 PLOS Biol. / 2023 under revision]

Cellular responses againt audible range of acoustic stimulation
Recent reseraches revealed acoustic waves as phpysical stimuli directly sensed at a cell-level. We established unique acoustic systems to emit audible range of acoutic waves to cultured cells, and try to reveal the acoustic effects on gene expression, metabolic regulation, and cell differentiation.
[2018 PLOS One]